Abstract
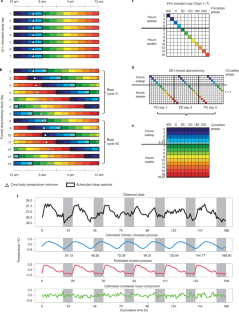
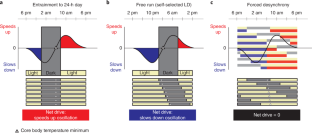
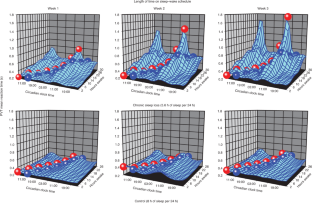
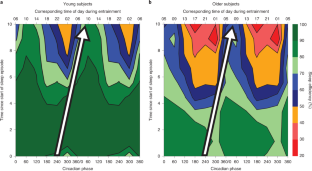
Circadian clocks drive cyclic variations in many aspects of physiology, but some daily variations are evoked by periodic changes in the environment or sleep–wake state and associated behaviors, such as changes in posture, light levels, fasting or eating, rest or activity and social interactions; thus, it is often important to quantify the relative contributions of these factors. Yet, circadian rhythms and these evoked effects cannot be separated under typical 24-h day conditions, because circadian phase and the length of time awake or asleep co-vary. Nathaniel Kleitman’s forced desynchrony (FD) protocol was designed to assess endogenous circadian rhythmicity and to separate circadian from evoked components of daily rhythms in multiple parameters. Under FD protocol conditions, light intensity is kept low to minimize its impact on the circadian pacemaker, and participants have sleep–wake state and associated behaviors scheduled to an imposed non-24-h cycle. The period of this imposed cycle, Τ, is chosen so that the circadian pacemaker cannot entrain to it and therefore continues to oscillate at its intrinsic period (τ, ~24.15 h), ensuring circadian components are separated from evoked components of daily rhythms. Here we provide detailed instructions and troubleshooting techniques on how to design, implement and analyze the data from an FD protocol. We provide two procedures: one with general guidance for designing an FD study and another with more precise instructions for replicating one of our previous FD studies. We discuss estimating circadian parameters and quantifying the separate contributions of circadian rhythmicity and the sleep–wake cycle, including statistical analysis procedures and an R package for conducting the non-orthogonal spectral analysis method that enables an accurate estimation of period, amplitude and phase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to Journal
Get full journal access for 1 year
99,00 €
only 8,25 € per issue
Tax calculation will be finalised during checkout.
Buy article
Get time limited or full article access on ReadCube.
$32.00
All prices are NET prices.

Data availability
All original datasets used in the preparation of this paper contain confidential or identifiable human data and are not publicly available due to human subject research regulations. De-identified sample datasets are available upon reasonable request and are subject to internal review and approval. Execution of a materials transfer agreement is required by our institution for transfer of data.
Code availability
SAS codes for data analyses and R codes for the NOSA program can be found at https://github.com/wwang4bwh/FD-NOSA.
References
-
Hunter, J. Of the heat of animals and vegetables. Philos. Trans. R. Soc. Lond. 68, 7–49 (1778).
-
Ogle, W. in St. George’s Hospital Reports (eds. Ogle, J. W. & Holmes, T.) 220–245 (John Churchill and Sons, 1866).
-
Benedict, F. G. Studies in body-temperature. I. Influence of the inversion of the daily routine; the temperature of night-workers. Am. J. Physiol. 11, 145–169 (1904).
-
Gibson, R. B. The effects of transposition of the daily routine on the rhythm of temperature variation. Am. J. Med. Sci. 129, 1048–1059 (1905).
-
Kleitman, N. Sleep and Wakefulness. (University of Chicago Press, 1939).
-
Aschoff, J., Gerecke, U. & Wever, R. Phasenbeziehungen zwischen den circadianen perioden der aktivitaet und der kerntemperatur beim menschen. Eur. J. Physiol. 295, 173–183 (1967).
-
Aschoff, J. Desynchronization and resynchronization of human circadian rhythms. Aerosp. Med. 40, 844–849 (1969).
-
Mills, J. N., Minors, D. S. & Waterhouse, J. M. Proceedings: Urinary and temperature rhythms on days of abnormal length. J. Physiol. 257, 54P–55P (1976).
-
Mills, J. N., Minors, D. S. & Waterhouse, J. M. The physiological rhythms of subjects living on a day of abnormal length. J. Physiol. 268, 803–826 (1977).
-
Czeisler, C. A. Human circadian physiology: internal organization of temperature, sleep-wake, and neuroendocrine rhythms monitored in an environment free of time cues. Ph.D. thesis, Stanford University (1978).
-
Schulz, H., Bes, E. & Jobert, M. in Sleep–Wake Disorders (eds. Meier-Ewert, K. & Okawa, M.) (Springer, 1997).
-
Klerman, E. B., Dijk, D. J., Kronauer, R. E. & Czeisler, C. A. Simulations of light effects on the human circadian pacemaker: implications for assessment of intrinsic period. Am. J. Physiol. 270, R271–R282 (1996).
-
Aschoff, J. & Wever, R. Spontanperiodik des menschen bei ausschluss aller zeitgeber. Die Naturwissenschaften 49, 337–342 (1962).
-
Aschoff, J., Gerecke, U. & Wever, R. Desynchronization of human circadian rhythms. Jpn. J. Physiol. 17, 450–457 (1967).
-
Sulzman, F. M., Fuller, C. A. & Moore-Ede, M. C. Spontaneous internal desynchronization of circadian rythms in the squirrel monkey. Comp. Biochem. Physiol. A Physiol. 58, 63–67 (1977).
-
Czeisler, C. A. & Jewett, M. E. in Handbook of Sleep Disorders (ed. Thorpy, M. J.) 117–137 (Marcel Dekker, 1990).
-
Pittendrigh, C. S. & Daan, S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J. Comp. Physiol. A 106, 291–331 (1976).
-
Carskadon, M. A. & Dement, W. C. Sleep studies on a 90-minute day. Electroencephalogr. Clin. Neurophysiol. 39, 145–155 (1975).
-
Lavie, P. & Scherson, A. Ultrashort sleep-waking schedule. I. Evidence of ultradian rhythmicity in ‘sleep ability’. Electroencephalogr. Clin. Neurophysiol. 52, 163–174 (1981).
-
Lavie, P. Ultrashort sleep-waking schedule III. “Gates” and “forbidden zones” for sleep. Electroencephalogr. Clin. Neurophysiol. 63, 414–425 (1986).
-
Haimov, I. & Lavie, P. Circadian characteristics of sleep propensity function in healthy elderly: a comparison with young adults. Sleep 20, 294–300 (1997).
-
Åkerstedt, T., Hume, K., Minors, D. & Waterhouse, J. Experimental separation of time of day and homeostatic influences on sleep. Am. J. Physiol. 274, R1162–R1168 (1998).
-
Kripke, D. F. et al. Circadian phase in adults of contrasting ages. Chronobiol. Int. 22, 695–709 (2005).
-
Eastman, C. I., Suh, C., Tomaka, V. A. & Crowley, S. J. Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci. Rep. 5, 8381 (2015).
-
Boivin, D. B. et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch. Gen. Psychiatry 54, 145–152 (1997).
-
Waterhouse, J. et al. Light of domestic intensity produces phase shifts of the circadian oscillator in humans. Neurosci. Lett. 245, 97–100 (1998).
-
Cajochen, C., Wyatt, J. K., Czeisler, C. A. & Dijk, D. J. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience 114, 1047–1060 (2002).
-
Cohen, D. A. et al. Uncovering residual effects of chronic sleep loss on human performance. Sci. Transl. Med. 2, 14ra13 (2010).
-
Czeisler, C. A. et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284, 2177–2181 (1999).
-
Duffy, J. F. et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl Acad. Sci. USA 108, 15602–15608 (2011).
-
Hull, J. T., Klerman, E. B., Dijk, D. J., Czeisler, C. A. & Lockley, S. W. Human circadian period of NLP blind individual with no conscious light perception assessed under field and forced desynchrony conditions. In 72nd Cold Spring Harbor Laboratory Symposium: Clocks & Rhythms (eds. Stillman, B., Stewart D., & Grodzicker, T.) 65 (Cold Spring Harbor Laboratory, 2007).
-
Dijk, D. J. & Czeisler, C. A. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J. Neurosci. 15, 3526–3538 (1995).
-
Wyatt, J. K., Ritz-De Cecco, A., Czeisler, C. A. & Dijk, D. J. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am. J. Physiol. Regul. Integr. Comp. Physiol. 277, R1152–R1163 (1999).
-
Dijk, D.-J., Duffy, J. F. & Czeisler, C. A. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep 24, 565–577 (2001).
-
Dijk, D.-J., Duffy, J. F., Riel, E., Shanahan, T. L. & Czeisler, C. A. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J. Physiol. 516, 611–627 (1999).
-
Dijk, D.-J., Duffy, J. F. & Czeisler, C. A. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 17, 285–311 (2000).
-
Duffy, J. F., Rimmer, D. W. & Czeisler, C. A. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav. Neurosci. 115, 895–899 (2001).
-
Wright, K. P. Jr., Hull, J. T. & Czeisler, C. A. Relationship between alertness, performance, and body temperature in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R1370–R1377 (2002).
-
Münch, M. et al. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol. Aging 26, 1307–1319 (2005).
-
Wyatt, J. K., Dijk, D.-J., Cecco, A. R.-D., Ronda, J. M. & Czeisler, C. A. Sleep-facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep 29, 609–618 (2006).
-
Cain, S. W., Rimmer, D. W., Duffy, J. F. & Czeisler, C. A. Exercise distributed across day and night does not alter circadian period in humans. J. Biol. Rhythms 22, 534–541 (2007).
-
O’Donnell, D. et al. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. J. Sleep. Res. 18, 254–263 (2009).
-
Lee, J. H. et al. Neurobehavioral performance in young adults living on a 28-h day for 6 weeks. Sleep 32, 905–913 (2009).
-
Silva, E. J., Wang, W., Ronda, J. M., Wyatt, J. K. & Duffy, J. F. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep 33, 481–490 (2010).
-
Munch, M., Silva, E. J., Ronda, J. M., Czeisler, C. A. & Duffy, J. F. EEG sleep spectra in older adults across all circadian phases during NREM sleep. Sleep 33, 389–401 (2010).
-
Duffy, J. F., Lowe, A. S., Silva, E. J. & Winkelman, J. W. Periodic limb movements in sleep exhibit a circadian rhythm that is maximal in the late evening/early night. Sleep. Med. 12, 83–88 (2011).
-
Santhi, N. et al. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl Acad. Sci. USA 113, E2730–E2739 (2016).
-
Lee, M. L. et al. Fragmentation of rapid eye movement and nonrapid eye movement sleep without total sleep loss impairs hippocampus-dependent fear memory consolidation. Sleep 39, 2021–2031 (2016).
-
McHill, A. W., Hull, J. T., Wang, W., Czeisler, C. A. & Klerman, E. B. Chronic sleep curtailment, even without extended (>16-h) wakefulness, degrades human vigilance performance. Proc. Natl Acad. Sci. USA 115, 6070–6075 (2018).
-
Zitting, K. M. et al. Young adults are more vulnerable to chronic sleep deficiency and recurrent circadian disruption than older adults. Sci. Rep. 8, 11052 (2018).
-
Zitting, K. M. et al. Human resting energy expenditure varies with circadian phase. Curr. Biol. 28, 3685–3690.e3 (2018).
-
Grady, S., Aeschbach, D., Wright, K. P. Jr. & Czeisler, C. A. Effect of modafinil on impairments in neurobehavioral performance and learning associated with extended wakefulness and circadian misalignment. Neuropsychopharmacology 35, 1910–1920 (2010).
-
Wyatt, J. K., Cajochen, C., Ritz-De Cecco, A., Czeisler, C. A. & Dijk, D. J. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep 27, 374–381 (2004).
-
Weaver, D. R. The suprachiasmatic nucleus: a 25-year retrospective. J. Biol. Rhythms 13, 100–112 (1998).
-
Shanahan, T. L. Circadian physiology and the plasma melatonin rhythm in humans. M.D. thesis, Harvard Medical School (1995).
-
Scheer, F. A., Wright, K. P. Jr., Kronauer, R. E. & Czeisler, C. A. Plasticity of the intrinsic period of the human circadian timing system. PLoS One 2, e721 (2007).
-
Czeisler, C. A. et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science 233, 667–671 (1986).
-
Duffy, J. F. & Dijk, D. J. Getting through to circadian oscillators: why use constant routines? J. Biol. Rhythms 17, 4–13 (2002).
-
Dijk, D. J. & Duffy, J. F. Novel approaches for assessing circadian rhythmicity in humans: a review. J. Biol. Rhythms 35, 421–438 (2020).
-
Wright, K. P. Jr., Hughes, R., Kronauer, R. E., Dijk, D. J. & Czeisler, C. A. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc. Natl Acad. Sci. USA 98, 14027–14032 (2001).
-
Banich, M. T. et al. fMri studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J. Cogn. Neurosci. 12, 988–1000 (2000).
-
Wright, K. P. Jr., Gronfier, C., Duffy, J. F. & Czeisler, C. A. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. Rhythms 20, 168–177 (2005).
-
Gronfier, C., Wright, K. P. Jr., Kronauer, R. E. & Czeisler, C. A. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc. Natl Acad. Sci. USA 104, 9081–9086 (2007).
-
Kronauer, R. E., Czeisler, C. A., Pilato, S. F., Moore-Ede, M. C. & Weitzman, E. D. Mathematical model of the human circadian system with two interacting oscillators. Am. J. Physiol. 242, R3–R17 (1982).
-
Borbély, A. A. A two process model of sleep regulation. Hum. Neurobiol. 1, 195–204 (1982).
-
Daan, S., Beersma, D. G. M. & Borbély, A. A. Timing of human sleep: recovery process gated by a circadian pacemaker. Am. J. Physiol. 246, R161–R183 (1984).
-
Dijk, D. J. & Czeisler, C. A. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci. Lett. 166, 63–68 (1994).
-
Duffy, J. F., Zitting, K. M. & Czeisler, C. A. The case for addressing operator fatigue. Rev. Hum. Factors Ergon. 10, 29–78 (2015).
-
Muto, V. et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science 353, 687–690 (2016).
-
Stepanski, E. J. & Wyatt, J. K. Use of sleep hygiene in the treatment of insomnia. Sleep. Med. Rev. 7, 215–225 (2003).
-
Eastman, C. I., Norwood, A. A., Velez, S. L., Paech, G. M. & Crowley, S. J. 0713 SEX AND THE FORBIDDEN ZONE. Sleep 40, A264 (2017).
-
Crowley, S. J. & Eastman, C. I. 0248 Sleep and the Forbidden Zone: Trouble for Teens. Sleep 41, A96 (2018).
-
Johnson, M. P. et al. Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J. Sleep. Res. 1, 24–29 (1992).
-
Edgar, D. M., Dement, W. C. & Fuller, C. A. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J. Neurosci. 13, 1065–1079 (1993).
-
Franken, P. & Dijk, D. J. Circadian clock genes and sleep homeostasis. Eur. J. Neurosci. 29, 1820–1829 (2009).
-
Klerman, E. B. in Zeitgebers, Entrainment and Masking of the Circadian System Sapporo Symposium (eds. Honma, K. & Honma, S.) 155–169 (Hokkaido Univ. Press, 2001).
-
Kitamura, S. et al. Intrinsic circadian period of sighted patients with circadian rhythm sleep disorder, free-running type. Biol. Psychiatry 73, 63–69 (2013).
-
Carskadon, M. A., Labyak, S. E., Acebo, C. & Seifer, R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci. Lett. 260, 129–132 (1999).
-
Crowley, S. J. & Eastman, C. I. Free-running circadian period in adolescents and adults. J. Sleep. Res. 27, e12678 (2018).
-
Kantermann, T. & Eastman, C. I. Circadian phase, circadian period and chronotype are reproducible over months. Chronobiol. Int. 35, 280–288 (2018).
-
Eastman, C. I., Tomaka, V. A. & Crowley, S. J. Circadian rhythms of European and African-Americans after a large delay of sleep as in jet lag and night work. Sci. Rep. 6, 36716 (2016).
-
Eastman, C. I., Tomaka, V. A. & Crowley, S. J. Sex and ancestry determine the free-running circadian period. J. Sleep. Res. 26, 547–550 (2017).
-
Eastman, C. I., Molina, T. A., Dziepak, M. E. & Smith, M. R. Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans). Chronobiol. Int. 29, 1072–1077 (2012).
-
Hiddinga, A. E., Beersma & Van Den Hoofdakker, R. H. Endogenous and exogenous components in the circadian variation of core body temperature in humans. J. Sleep. Res. 6, 156–163 (1997).
-
Hasan, S. et al. Assessment of circadian rhythms in humans: comparison of real-time fibroblast reporter imaging with plasma melatonin. FASEB J. 26, 2414–2423 (2012).
-
Hida, A. et al. In vitro circadian period is associated with circadian/sleep preference. Sci. Rep. 3, 2074 (2013).
-
Silva, E. J. & Duffy, J. F. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav. Neurosci. 122, 928–935 (2008).
-
Scheer, F. A., Shea, T. J., Hilton, M. F. & Shea, S. A. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J. Biol. Rhythms 23, 353–361 (2008).
-
Burke, T. M., Scheer, F. A., Ronda, J. M., Czeisler, C. A. & Wright, K. P. Jr. Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J. Sleep. Res. 24, 364–371 (2015).
-
McHill, A. W. et al. Chronic sleep restriction greatly magnifies performance decrements immediately after awakening. Sleep 42, zsz032 (2019).
-
Lazar, A. S. et al. Circadian period and the timing of melatonin onset in men and women: predictors of sleep during the weekend and in the laboratory. J. Sleep. Res. 22, 155–159 (2013).
-
Weitzman, E. D. et al. Effects of a prolonged 3-hour sleep-wake cycle on sleep stages, plasma cortisol, growth hormone, and body temperature in man. J. Clin. Endocrinol. Metab. 38, 1018–1030 (1974).
-
Strijkstra, A. M., Meerlo, P. & Beersma, D. G. M. Forced desynchrony of circadian rhythms of body temperature and activity in rats. Chronobiol. Int. 16, 431–440 (1999).
-
Koorengevel, K. M., Beersma, D. G., den Boer, J. A. & van den Hoofdakker, R. H. Mood regulation in seasonal affective disorder patients and healthy controls studied in forced desynchrony. Psychiatry Res. 117, 57–74 (2003).
-
Yasenkov, R. & Deboer, T. Circadian regulation of sleep and the sleep EEG under constant sleep pressure in the rat. Sleep 33, 631–641 (2010).
-
Pomplun, M. et al. The effects of circadian phase, time awake, and imposed sleep restriction on performing complex visual tasks: evidence from comparative visual search. J. Vis. 12, 14 (2012).
-
Wotus, C. et al. Forced desynchrony reveals independent contributions of suprachiasmatic oscillators to the daily plasma corticosterone rhythm in male rats. PLoS One 8, e68793 (2013).
-
Wu, L. J., Acebo, C., Seifer, R. & Carskadon, M. A. Sleepiness and cognitive performance among younger and older adolescents across a 28-hour forced desynchrony protocol. Sleep 38, 1965–1972 (2015).
-
Walsh, L., McLoone, S., Ronda, J., Duffy, J. F. & Czeisler, C. A. Non-contact pressure-based sleep/wake discrimination. IEEE Trans. Biomed. Eng. 64, 1750–1760 (2017).
-
Qian, J., Scheer, F. A., Hu, K. & Shea, S. A. The circadian system modulates the rate of recovery of systolic blood pressure after exercise in humans. Sleep 43, zsz253 (2020).
-
Morgan, L. et al. Effects of the endogenous clock and sleep time on melatonin, insulin, glucose and lipid metabolism. J. Endocrinol. 157, 443–451 (1998).
-
Scheer, F. A., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458 (2009).
-
Scheer, F. A. et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc. Natl Acad. Sci. USA 107, 20541–20546 (2010).
-
Buxton, O. M. et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 4, 129ra143 (2012).
-
Swanson, C. M. et al. Bone turnover markers after sleep restriction and circadian disruption: a mechanism for sleep-related bone loss in humans. J. Clin. l Endocrinol. Metab. 102, 3722–3730 (2017).
-
Yuan, R. K. et al. Fasting blood triglycerides vary with circadian phase in both young and older people. Physiol. Rep. 8, e14453 (2020).
-
Scheer, F. et al. The endogenous circadian system worsens asthma at night independent of sleep and other daily behavioral or environmental cycles. Proc. Natl Acad. Sci. USA 118, e2018486118 (2021).
-
Chellappa, S. L. et al. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci. Adv. 7, eabg9910 (2021).
-
Chellappa, S. L. et al. Proof-of-principle demonstration of endogenous circadian system and circadian misalignment effects on human oral microbiota. FASEB J. 36, e22043 (2022).
-
Zitting, K. M. et al. Chronic circadian disruption on a high-fat diet impairs glucose tolerance. Metabolism 130, 155158 (2022).
-
Archer, S. N. et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc. Natl Acad. Sci. USA 111, E682–E691 (2014).
-
Chang, A. M., Buch, A. M., Bradstreet, D. S., Klements, D. J. & Duffy, J. F. Human diurnal preference and circadian rhythmicity are not associated with the CLOCK 3111C/T gene polymorphism. J. Biol. Rhythms 26, 276–280 (2011).
-
Chang, A. M. et al. Chronotype genetic variant in PER2 is associated with intrinsic circadian period in humans. Sci. Rep. 9, 5350 (2019).
-
Micic, G., Lovato, N., Ferguson, S. A., Burgess, H. J. & Lack, L. Circadian tau differences and rhythm associations in delayed sleep-wake phase disorder and sighted non-24-hour sleep-wake rhythm disorder. Sleep 44, zsaa132 (2021).
-
Ben-Hamo, M. et al. Circadian forced desynchrony of the master clock leads to phenotypic manifestation of depression in rats. eNeuro 3, ENEURO.0237-16.2016 (2016).
-
Laing, E. E. et al. Blood transcriptome based biomarkers for human circadian phase. Elife 6, e20214 (2017).
-
Moses, J., Naitoh, P. & Johnson, L. C. The REM cycle in altered sleep/wake schedules. Psychophysioly 15, 569–575 (1978).
-
Micic, G. et al. Circadian melatonin and temperature taus in delayed sleep-wake phase disorder and non-24-hour sleep-wake rhythm disorder patients: an ultradian constant routine study. J. Biol. Rhythms 31, 387–405 (2016).
-
Czeisler, C. A. & Dijk, D. J. in Handbook of Behavioral Neurobiology: Circadian Clocks (eds. Takahashi, J. S., Turek, F. W., & Moore, R. Y.) 531–569 (Plenum, 2001).
-
Aschoff, J. Circadian rhythms in man: a self-sustained oscillator with an inherent frequency underlies human 24-hour periodicity. Science 148, 1427–1432 (1965).
-
Czeisler, C. A., Weitzman, E. D., Moore-Ede, M. C., Zimmerman, J. C. & Knauer, R. S. Human sleep: its duration and organization depend on its circadian phase. Science 210, 1264–1267 (1980).
-
Brown, E. N. & Czeisler, C. A. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J. Biol. Rhythms 7, 177–202 (1992).
-
Weitzman, E. D., Czeisler, C. A., Zimmerman, J. C., Ronda, J. M. & Knauer, R. S. in Disorders of Sleeping and Waking: Indications and Techniques (ed. Guilleminault, C.) 297–329 (Addison-Wesley, 1982).
-
Dinges, D. F. & Powell, J. W. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 17, 652–655 (1985).
-
Enright, J. T. in Circadian Clocks (ed. Aschoff, J.) 112–124 (North-Holland, 1965).
-
Brown, E. N., Solo, V. & Zhang, Z. in Methods in Enzymology, Numerical Computer Methods Vol. 383 (eds. Brand, L. & Johnson, M. L.) 383–405 (Academic, 2004).
-
Sargent, C., Darwent, D., Ferguson, S. A., Kennaway, D. J. & Roach, G. D. Sleep restriction masks the influence of the circadian process on sleep propensity. Chronobiol. Int. 29, 565–571 (2012).
-
Buxton, O. M., Lee, C. W., L’Hermite-Balériaux, M., Turek, F. W. & Van Cauter, E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R714–R724 (2003).
-
Dijk, D. J., Duffy, J. F. & Czeisler, C. A. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J. Sleep. Res. 1, 112–117 (1992).
-
Jewett, M. E. Models of Circadian and Homeostatic Regulation of Human Performance and Alertness. Ph.D. thesis, Harvard University (1997).
-
Krahn, L. E. et al. Recommended protocols for the Multiple Sleep Latency Test and Maintenance of Wakefulness Test in adults: guidance from the American Academy of Sleep Medicine. J. Clin. Sleep. Med. 17, 2489–2498 (2021).
-
Fraser, S., Cowen, P., Franklin, M., Franey, C. & Arendt, J. Direct radioimmunoassay for melatonin in plasma. Clin. Chem. 29, 396–397 (1983).
-
Yie, S. M., Johansson, E. & Brown, G. M. Competitive solid-phase enzyme immunoassay for melatonin in human and rat serum and rat pineal gland. Clin. Chem. 39, 2322–2325 (1993).
-
de Almeida, E. A. et al. Measurement of melatonin in body fluids: standards, protocols and procedures. Childs Nerv. Syst. 27, 879–891 (2011).
-
Kennaway, D. J. A critical review of melatonin assays: past and present. J. Pineal Res. 67, e12572 (2019).
-
Laird, N. M. & Ware, J. H. Random-effects models for longitudinal data. Biometrics 38, 963–974 (1982).
-
Brown, E. N., Choe, Y., Luithardt, H. & Czeisler, C. A. A statistical model of the human core-temperature circadian rhythm. Am. J. Physiol. Endocrinol. Metab. 279, E669–E683 (2000).
-
Rechtschaffen, A. & Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects (US Government Printing Office, 1968).
-
Cajochen, C., Wyatt, J. K., Czeisler, C. A., Bonikowska, M. & Dijk, D. J. Non-linear interaction between the circadian and homeostatic modulation of slow eye movements during wakefulness in humans. J. Sleep. Res. 9, 58 (2000).
-
Wyatt, R. J., Fram, D. H., Kupfer, D. J. & Snyder, F. Total prolonged drug-induced REM sleep suppression in anxious- depressed patients. Arch. Gen. Psychiatry 24, 145–155 (1971).
-
Aeschbach, D. et al. Two circadian rhythms in the human electroencephalogram during wakefulness. Am. J. Physiol. 277, R1771–R1779 (1999).
-
Smith, M. E., McEvoy, L. K. & Gevins, A. The impact of moderate sleep loss on neurophysiologic signals during working-memory task performance. Sleep 25, 784–794 (2002).
-
Gillberg, M., Kecklund, G. & Åkerstedt, T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep 17, 236–241 (1994).
-
Kennaway, D. J. Measuring melatonin by immunoassay. J. Pineal Res. 69, e12657 (2020).
Acknowledgements
The authors acknowledge Drs. J.S. Allan, D.B. Boivin, S.W. Cain, C. Cajochen, A.M. Chang, D. Cohen, S.P. Grady, C. Gronfier, J.T. Hull, M.Y. Münch, D.W. Rimmer, A.W. McHill and K.D. Scheuermaier, each of whom carried out FD studies when they were a trainee in the laboratory. The authors acknowledge Seungyeop Kang for help with R programming. The authors also acknowledge the critical contributions of the late Richard E. Kronauer to the development of the FD protocol and its analyses. The FD studies and data described here were collected in the Environmental Scheduling Facility (ESF) and the Intensive Physiological Monitoring (IPM) Unit at the Brigham and Women’s Hospital. Both facilities were part of the BWH General Clinical Research Center (supported by National Institutes of Health (NIH) Grant M01 RR002635), and the IPM was part of the Harvard Clinical and Translational Science Center (supported by NIH Award UL1 RR025758 and financial contributions from the Brigham and Women’s Hospital and from Harvard University and its affiliated academic health care centers). The development of the FD protocol and NOSA program and carrying out the FD studies described here were supported by NIH Grants P01 AG09975, R01 HL080978, R01 HL52992, R21 AT02571, U01 AG12642 and T32 HL07901; by National Aeronautics and Space Administration Grants NAS9-19435 and NAG 5-3952 and NASA Cooperative Agreement NCC9-58 with the National Space Biomedical Research Institute; and by Air Force Office of Scientific Research Grants F49620-94-1-0398, F49620-95-1-0388, FA9550-06-0080 and F49620-00-1-0266.
Author information
Authors and Affiliations
Contributions
C.A.C. developed the concept and designed the FD protocol as implemented in our laboratory. J.M.R. designed and built the data-collection hardware and software, the protocol-scheduling software and the data-management system used in our laboratory. E.N.B. designed the statistical model, analysis and computational framework for the NOSA procedure. E.N.B., J.F.M., D.-J.D., and C.A.C. contributed to the implementation of the computational framework for the NOSA procedure. C.A.C., D.-J.D., E.B.K., and M.A.S.H. carried out simulations to determine the impact of light levels on period estimates from FD protocols and free runs. D.-J.D. developed the analyses framework for the separation of circadian and sleep–wake contributions to sleep and performance. R.K.Y., K.-M.Z., J.K.W., F.A.J.L.S., K.P.W., E.B.K., J.F.D. and D.-J.D. carried out FD studies and analyses. W.W. re-analyzed FD data presented here and wrote and updated the NOSA program in R for sharing. W.W., R.K.Y. and K.-M.Z. created figures for the manuscript. All authors contributed to drafting, writing and/or editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
W.W. serves as a consultant for the National Sleep Foundation. E.B.K. receives support from the Gordon Research Conference, the Sleep Research Society, the Santa Fe Institute and DGSM (German Sleep Society); she has consultancies for Circadian Therapeutics, the National Sleep Foundation, the Puerto Rico Science Technology Trust and Sanofi-Genzyme; her partner owns Chronsulting. C.A.C. reports grants/contracts to BWH from Dayzz Live Well, Ltd., Delta Airlines, FAA, Jazz Pharmaceuticals, NHLBI, NIA, NIOSH, NASA, Puget Sound Pilots, Regeneron Pharmaceuticals/Sanofi and DOD; reports grants/gifts to Monash University from the CDC Foundation, with funding from BNY Mellon, and WHOOP; is/was a paid consultant or received lecture fees from Emory University, Inselspital Bern, UCLA, the Institute of Digital Media and Child Development, the Klarman Family Foundation, the National Council for Mental Wellbeing, the National Sleep Foundation, Physician’s Seal, the SRS Foundation, Tencent Holdings, Teva Pharma Australia, With Deep and Vanda Pharmaceuticals Inc., in which he holds an equity interest; received travel support from the Aspen Brain Institute, the Bloomage International Investment Group, UK Biotechnology and Biological Sciences Research Council, Bouley Botanicals, the Dr. Stanley Ho Medical Development Foundation, EBRS, the German National Academy of Sciences (Leopoldina), the National Safety Council, the National Sleep Foundation, Stanford Medical School, Tencent Holdings and Vanda Pharmaceuticals Inc.; receives research/education support through BWH from Arbor Pharmaceuticals, Avadel Pharmaceuticals, Beijing Zhaode Healthcare Management Consulting Co., Bryte, Alexandra Drane, Eisai, Harmony Biosciences, Jazz Pharmaceuticals, Johnson & Johnson, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, NeuroCare, Inc., Optum, Philips Respironics, Regeneron Pharmaceuticals, Regional Home Care, ResMed, San Francisco Bar Pilots, Sanofi, Schneider, Simmons, Sleep Cycle, Sleep Number, Sysco, Teva Pharmaceuticals Industries and Vanda Pharmaceuticals Inc.; is/was an expert witness in legal cases, including those involving Advanced Power Technologies, Aegis Chemical Solutions LLC, Amtrak, Casper Sleep Inc., C&J Energy Services, Catapult Energy Services Group, Covenant Testing Technologies, the Dallas Police Association, Enterprise Rent-A-Car, Espinal Trucking/Eagle Transport Group/Steel Warehouse Inc., FedEx, Greyhound Lines Inc./Motor Coach Industries/FirstGroup America, PAR Electrical Contractors Inc., Product & Logistics Services LLC/Schlumberger Technology Corp/Gelco Fleet Trust, Puckett Emergency Medical Services LLC, Puget Sound Pilots, Union Pacific Railroad, United Parcel Service and Vanda Pharmaceuticals Inc.; serves as the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc.; and receives royalties from McGraw Hill and from Philips Respironics for the Actiwatch-2 and Actiwatch Spectrum devices. C.A.C.’s interests were reviewed and are managed by the BWH and MGB in accordance with their conflict of interest policies. D.-J.D. was a paid consultant to F. Hoffmann-La Roche Ltd., Pfizer Inc., Eli Lilly and Company, Novo Nordisk A/S and Ono Pharma UK Ltd. and has received research support from Janssen Research & Development LLC, Eli Lilly and Company and GW Pharma. M.A.S.H. has provided paid limited consulting for The MathWorks, Inc. K.P.W. reports research support/donated materials: DuPont Nutrition & Biosciences, Grain Processing Corporation, and Friesland Campina Innovation Centre and being a consultant to and/or receiving personal fees from Circadian Therapeutics, Inc., Circadian Biotherapies, Inc., Philips, Inc, and U.S. Army Medical Research and Materiel Command - Walter Reed Army Institute of Research, outside the submitted work.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Czeisler, C. A. et al. Science 284, 2177–2181 (1999): https://doi.org/10.1126/science.284.5423.2177
Dijk, D. J. et al. J. Neurosci. 15, 3526–3538 (1995): https://doi.org/10.1523/JNEUROSCI.15-05-03526.1995
Scheer, F. A. et al. PLoS One 2, e721 (2007): https://doi.org/10.1371/journal.pone.0000721
Cohen, D. A. et al. Sci. Transl. Med. 2, 14ra13 (2010): https://doi.org/10.1126/scitranslmed.3000458
Duffy, J. F. et al. Proc. Natl Acad. Sci. USA 108, 15602–15608 (2011): https://doi.org/10.1073/pnas.1010666108
Key data used in this protocol
Dijk, D. J. et al. J. Physiol. 516, 611–627 (1999): https://doi.org/10.1111/j.1469-7793.1999.0611v.x
Duffy, J. F. et al. Proc. Natl Acad. Sci. USA 108, 15602–15608 (2011): https://doi.org/10.1073/pnas.1010666108
Cohen, D. A. et al. Sci. Transl. Med. 2, 14ra13 (2010): https://doi.org/10.1126/scitranslmed.3000458
Supplementary information
Supplementary Information
Supplementary Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Yuan, R.K., Mitchell, J.F. et al. Desynchronizing the sleep–wake cycle from circadian timing to assess their separate contributions to physiology and behaviour and to estimate intrinsic circadian period. Nat Protoc (2022). https://ift.tt/1m9Ycx4
-
Received:
-
Accepted:
-
Published:
-
DOI: https://ift.tt/1m9Ycx4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
"cycle" - Google News
November 14, 2022 at 11:01PM
https://ift.tt/HaN9Awo
Desynchronizing the sleep–wake cycle from circadian timing to assess their separate contributions to physiology and behaviour and to estimate intrinsic circadian period - Nature.com
"cycle" - Google News
https://ift.tt/HRSMuIk
https://ift.tt/LIjTyhJ
Bagikan Berita Ini














0 Response to "Desynchronizing the sleep–wake cycle from circadian timing to assess their separate contributions to physiology and behaviour and to estimate intrinsic circadian period - Nature.com"
Post a Comment